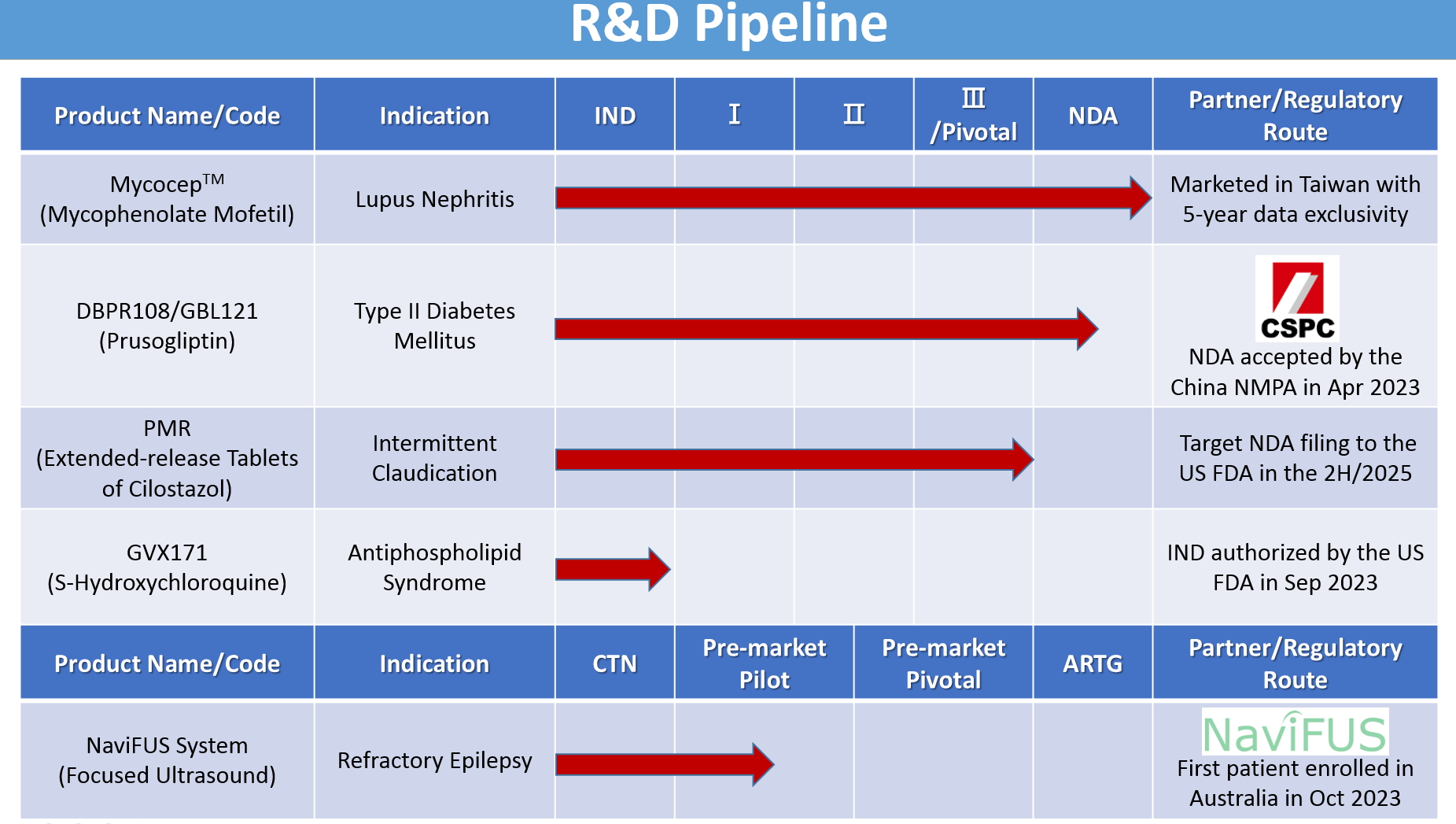
|
Genovate Biotechnology’s ongoing research and development focuses on finding treatments for metabolic, cardiovascular, neurodegenerative, and autoimmune diseases. The main research and development projects are as follows. |
1.MycocepTM (Mycophenolate Mofetil)
Brief description: MycocepTM is Genovate’s proprietary formulation of mycophenolate mofetil (MMF), which has emerged as the first-line maintenance therapy, combined with steroids, for the treatment of lupus nephritis (LN). Clinically, MMF has shown superior efficacy with fewer serious adverse events compared to another standard immunosuppressant azathioprine for LN treatment. Based on the clinical study conducted in Taiwan, Genovate’s MycocepTM was approved in Taiwan in 2018 with 5-year data exclusivity, providing an effective treatment option for patients with LN. NCT02949349

Systemic lupus erythematosus (SLE) is an autoimmune disease that can affect any organ in the body, with the kidneys being one of the most commonly affected organs. Severe lupus nephritis (LN), if not well treated, can progress to end-stage renal disease (ESRD) and even death.
Among Chinese patients, the prevalence of kidney disease 5 years after the initial diagnosis of SLE is 49%, with clinical manifestations including proteinuria, hematuria, elevated serum creatinine levels, hypertension, and uremia. The 5-year and 10-year renal survival rates of Chinese patients with LN is 74%-84%, with approximately 10%-15% of patients with nephritis progressing to ESRD. Early detection of LN and timely, effective treatment would contribute to achieving higher rates of disease remission.
High-dose steroids combined with immunosuppressants or cytotoxic agents have been the mainstream treatments for LN. In recent years, however, mycophenolate mofetil (MMF), originally used as an immunosuppressant for transplantation, has shown significant efficacy in the induction and maintenance therapy of proliferative LN. The 2012 American College of Rheumatology treatment guidelines recommend maintenance therapy for LN as follows. (1) MMF at a dose of 1-2 gm/day or (2) azathioprine (AZA) at a dose of 2 mg/kg/day, with low-dose steroids as needed. MMF has emerged as the first-line, standard-of-care therapy for maintenance therapy of LN based on its superior efficacy compared to AZA, including delaying the time to treatment failure and fewer serious adverse events.
The costs of MMF for the treatment of LN had been reimbursed by the national health insurance in some Asian countries, including South Korea, Malaysia, and China, but not in Taiwan until 2018. Based on requests from the Taiwan Rheumatology Association and recommendations from the Taiwan Food and Drug Administration, Genovate completed a clinical study (NCT02949349) enrolling Taiwanese patients, and the study results showed that low-dose MMF (1.0-1.5 gm/day), combined with low-dose glucocorticoids (≤ 10 mg/day), was a safe and cost-effective maintenance therapy for LN. Genovate’s MMF formulation, marketed as Mycocep (250-mg capsules), was approved for this new indication and included in the reimbursement scheme in 2018, with 5-year data exclusivity in Taiwan, meeting the medical needs of Taiwanese SLE patients in a cost-effective way for them.
2.DBPR108/GBL121 (Prusogliptin)
Brief description: DBPR108, a new DPP-4 inhibitor, is being jointly developed by Genovate, its Taiwanese industry partners, and the National Health Research Institutes for the treatment of type II diabetes mellitus. Genovate has partnered with CSPC Pharmaceutical Group Limited for the development and commercialization of DBPR108 in China. DBPR108 aims to increase GLP-1 concentrations to control blood glucose, with benefits such as improved glucose tolerance, increased insulin secretion, and better insulin sensitivity. CSPC completed the clinical development and had its new drug application accepted by the National Medical Products Administration (NMPA) in China in April 2023. NCT04161430
Due in part to the modern lifestyle, the global diabetic population is rapidly increasing. Diabetes mellitus now ranks as the fourth leading cause of death worldwide, posing a serious threat to human health. Statisticians estimate that by 2025, the diabetic population will reach as high as 300 million people worldwide. The primary cause of diabetes is attributed to either insulin resistance or abnormal insulin secretion, leading to type II diabetes mellitus. Current treatments for diabetes mostly rely on oral hypoglycemic agents or insulin injections to control blood glucose levels. However, these treatment modalities have limited efficacy, poor tolerability, and often come with significant side effects, necessitating an urgent need for new treatment methods or drugs. Dipeptidyl peptidase-4 (DPP-4) inhibitors represent a novel and effective approach to treating type II diabetes mellitus. Under normal physiological conditions, DPP-4 rapidly degrades glucagon-like peptide-1 (GLP-1), a molecule that controls blood glucose levels, resulting in a half-life of only about one and a half minutes for GLP-1 in the human bloodstream. Currently, most of the GLP-1 agonists, such as exenatide, require administration via injection for diabetes treatment, which is inconvenient and often associated with side effects such as gastric distention and nausea, leading to poor patient acceptance of this class of therapeutic drugs. The development of DPP-4 inhibitors in recent years has aimed to inhibit DPP-4 activity, thereby increasing GLP-1 concentrations in the blood to achieve blood glucose control. The known advantages of DPP-4 inhibitors in treating diabetes include (1) improving glucose tolerance and increasing in vivo insulin secretion, (2) sensitively increasing the accumulation of active glucose-dependent insulinotropic polypeptide (GIP) and GLP-1, (3) improving β-cell response to glucose, and (4) improving insulin sensitivity in patients with type II diabetes. Among these new drugs, sitagliptin was the first on the market, receiving the U.S. Food and Drug Administration (FDA) approval at the end of 2006. Sitagliptin effectively lowers glycated hemoglobin (HbA1c), with very mild adverse reactions and fewer instances of weight gain and hypoglycemia. Preclinical studies of the novel anti-diabetic DPP-4 inhibitor “DBPR108” and its Phase I clinical trials under an investigational new drug application granted by the U.S. FDA have been successfully completed. This occurred under the “Facilitating Successful Biotechnology Investment Projects,” funded by the Taiwan Ministry of Science and Technology and executed jointly by the National Health Research Institutes and Genovate, along with its Taiwanese industry partners. Genovate’s collaborator, CSPC Pharmaceutical Group, Ltd., led the further clinical development of DBPR108 in China. CSPC has completed this phase and filed a new drug application with the National Medical Products Administration (NMPA) in China, which was accepted in April 2023. People with intermittent claudication experience muscle pain in the lower extremities after walking or exercising. Although it typically resolves upon resting, it tends to recur once the activity is resumed. The primary cause of intermittent claudication is insufficient blood flow and oxygen supply to the muscles of the lower extremities. This failure to meet the metabolic demands of the peripheral muscle tissue results in pain. The occurrence and location of claudication directly correlate with the degree and location of vascular narrowing. This disorder is classified as a peripheral arterial disease (PAD), which is a manifestation of systemic arterial sclerosis, often associated with other atherosclerotic diseases such as coronary artery disease and cerebrovascular disease. Risk factors for PAD include hypertension, hyperglycemia, hyperlipidemia, hyperuricemia, smoking, and so forth. When PAD leads to reduced arterial blood flow in the lower extremities, it can manifest as intermittent claudication, causing numbness in the legs or feet, severe pain even at rest, and difficulty in healing leg ulcers and wounds. PAD serves as a significant warning sign for cardiovascular events such as stroke and myocardial infarction, necessitating early intervention to reduce the risk of cardiovascular events. Currently, the U.S. Food and Drug Administration (FDA) has approved two drugs for the treatment of intermittent claudication, (1) pentoxifylline, approved in 1984 and (2) cilostazol, approved in 1999. The immediate-release form of cilostazol (cilostazol IR) was first marketed in Japan in 1988 and was approved for sale in Taiwan in 2000. Cilostazol is a reversible PDE-3 inhibitor with antiplatelet aggregation and vasodilation effects. Additionally, cilostazol improves blood lipid profiles (increasing HDL and decreasing TG levels). Clinically, cilostazol has shown good efficacy in preventing secondary strokes, notably reducing the rates of ischaemic stroke recurrence. Nevertheless, the results from eight placebo-controlled clinical trials indicated that 15.4% of patients early terminated cilostazol IR treatment because of adverse reactions. Headache, palpitations, and diarrhea were the most frequently reported adverse reactions among these patients. These adverse reactions were attributable to the high peak-to-trough variation of cilostazol’s concentration in the blood. Genovate is developing an extended-release tablet of cilostazol, PMR, to be administered once daily aiming to reduce drug concentration-dependent side effects and improve treatment compliance. The results of the new drug application (NDA)-enabling study conducted under U.S. investigational new drug have shown that the primary endpoints of the study were achieved. Once-daily PMR was demonstrated to be bioequivalent to twice-daily cilostazol IR. Genovate plans to submit the NDA for PMR under 505(b)(2) regulations to the U.S. FDA in the second half of 2025. Antiphospholipid syndrome (APS) is an autoimmune disorder where the immune system abnormally targets phospholipids, components of cell membranes, leading to the production of harmful antibodies. These antibodies cause a range of clinical manifestations including arterial and venous thrombosis, stroke, pulmonary hypertension, thrombocytopenia, and complications in pregnancy such as recurrent miscarriage, preterm delivery, fetal death, and preeclampsia. The prevalence of APS is approximately 40-50 cases per 100,000 individuals, with an annual incidence of 2-5 new cases per 100,000. The prevalence is five times greater in women than in men. The recurrence rate within 5 years is approximately 20%-30%, and the mortality rate for catastrophic APS can be as high as 50%. No medication or non-medication therapies have been approved for the treatment of APS. Current APS treatments include aspirin for initial arterial thrombosis prevention only and long-term use of vitamin K antagonists such as warfarin to prevent thrombosis recurrence. However, vitamin K antagonists require continuous monitoring and dosage increases due to the potential for resistance over time, creating a complicated treatment regimen. GVX171, developed by Genovate, is an (S)-(+)-stereoisomer of racemic hydroxychloroquine sulfate (HCQ). The current product on the market is a racemic mixture (1:1) of two enantiomers of HCQ, with some reports of irreversible retinal damage. This retinal adverse effect has been observed in long-term or high-dosage 4-aminoquinoline therapy for discoid, systemic lupus erythematosus, or rheumatoid arthritis in a dose-related manner. S-HCQ was found to be approximately 70% more active than the other enantiomer, R-HCQ. Additionally, preclinical studies showed that the accumulation of HCQ is enantioselective, suggesting that S-HCQ is expected to reduce concentration-dependent HCQ side effects, such as irreversible retinal damage and the cardiac risks of QT-interval prolongation. S-HCQ offers superior safety for long-term use, with reduced fluctuations in blood concentration, prolonged efficacy, and lower toxicity compared to racemic HCQ. Preclinical studies demonstrated that GVX171 exhibits greater efficacy in inhibiting the binding of anti-β2GPI antibodies to cell membrane phospholipids and reducing thrombus formation in APS mouse models compared to standard HCQ products. This new treatment is administered orally as a tablet and aims to provide a more effective and safer option for preventing and treating thrombosis in APS patients and decreasing the concentration-dependent HCQ side effects. Genovate has established a reliable, GMP-compliant manufacturing process for GVX171. The U.S. Food and Drug Administration approved Genovate’s investigational new drug application for the treatment of APS in 2022. Epilepsy is one of the most common neurological disorders, affecting more than 50 million people worldwide. Antiepileptic drugs serve as first-line treatment to reduce the excitability of the brain and lower the seizure frequency in epilepsy patients. Nevertheless, drug-resistant epilepsy (DRE) is present in up to 30% of patients with epilepsy. Resective surgery of the epileptogenic regions is the most effective option to treat patients with DRE. However, up to 60% of DRE patients are unsuitable for resective surgery due to the epileptogenic focus being located in the functional or deep tissues of the brain, or due to the inability to define a unique epileptogenic zone. Neuromodulation technology is an alternative treatment offering modulation of neuronal circuits for the management of DRE. Invasive neuromodulation techniques that are currently approved by the U.S. Food and Drug Administration for the treatment of epilepsy, such as deep brain stimulation (DBS), vagus nerve stimulation (VNS), and responsive neurostimulation (RNS), require complex neurosurgery and could cause serious side effects, such as infection, bleeding, non-target brain tissue damage, and misplacement due to normal tissue variability. Therefore, there is a need for new noninvasive treatment options for DRE. Focused ultrasound (FUS) is a novel, noninvasive, therapeutic technology with the potential to improve the quality of life and decrease the cost of care for patients with epilepsy. This method uses multiple ultrasound beams at either a low or high intensity to modulate neuronal activity or ablate neural tissue, respectively. Low-intensity focused ultrasound (LIFU) has been shown to attenuate the acute epileptic signals in animal models and has been evaluated in some clinical trials for epilepsy patients. Thus, LIFU has the potential to be a therapeutic option for patients with DRE. The NaviFUS System (a neuronavigation-guided focused ultrasound system) developed by NaviFUS Corporation uses LIFU to deliver transcranial burst-mode ultrasound energy to induce a neuromodulation effect in the specific area of the brain responsible for the clinical manifestations of epilepsy such as seizures. The first completed clinical trial of LIFU neuromodulation with the NaviFUS System for epilepsy was conducted at Taipei Veterans General Hospital to evaluate the safety and feasibility of the treatment in DRE patients (NCT03860298). The results showed that LIFU safely delivered to the seizure onset zone and modulated the neuronal activity by decreasing the spectral power in all frequency bands of stereo-electroencephalography (SEEG)signals with a dose-dependent effect. Additional ongoing clinical trials evaluating the safety and preliminary efficacy of LIFU neuromodulation in patients with DRE include two pilot studies conducted at Taipei Veterans General Hospital (NCT04999046 and NCT06492720) and a pilot study conducted at Stanford University School of Medicine, Brigham and Women’s Hospital, and the University of Virginia School of Medicine (NCT06388707). Genovate, in collaboration with NaviFUS Corporation, has been conducting a pre-market pilot study using LIFU to treat DRE patients with the NaviFUS System at The Alfred in Australia since 2023 (NCT05947656 / ACTRN12623000382673). 
3.PMR (Extended-release Tablets of Cilostazol)
Brief description: PMR is an extended-release formulation of cilostazol developed by Genovate for the treatment of intermittent claudication. By lowering peak-to-trough variation in blood concentrations caused by the immediate-release formulation of cilostazol (cilostazol IR), it aims to reduce cilostazol’s concentration-dependent side effects, mainly headaches, palpitations, and diarrhea. Genovate has completed the new drug application (NDA)-enabling clinical trial in the U.S., demonstrating that once-daily PMR is bioequivalent to twice-daily cilostazol IR. Genovate plans to submit a NDA for PMR under 505(b)(2) regulations to the U.S. Food and Drug Administration in the second half of 2025.NCT06167265

4.GVX171 (S-Hydroxychloroquine)
Brief description: Genovate is currently developing GVX171, an (S)-(+)-stereoisomer of racemic hydroxychloroquine (HCQ), for the treatment of antiphospholipid syndrome (APS). To date, there are no medication or non-medication therapies approved for the treatment of APS. With GVX171, Genovate aims to provide the most potent HCQ derivative with the least tissue accumulation, in hopes of increasing its bioavailability and decreasing the concentration-dependent HCQ side effects such as the irreversible retinal damage and the cardiac risks of QT-interval prolongation. In 2022, the U.S. Food and Drug Administration granted Genovate’s investigational new drug application for the treatment of patients with APS.

5.NaviFUS System
Brief description: NaviFUS System, developed by NaviFUS Corporation, is a novel, neuronavigation-guided focused ultrasound (FUS) medical device that noninvasively delivers FUS energy into deep tissues in the brain for the treatment of central nervous system diseases. Low-intensity focused ultrasound (LIFU) has been shown to have a neuromodulation effect in patients with drug-resistant epilepsy in several clinical studies. Genovate, in collaboration with NaviFUS Corporation, has been conducting a pre-market pilot study using LIFU to treat patients with drug-resistant epilepsy using the NaviFUS System in Australia since 2023.NCT05947656 / ACTRN12623000382673


 繁中
繁中