Genovate Biotechnology Co., Ltd.;
Genovate was founded in 1993 in the Hsinchu Science Industrial Park, Taiwan. The company’s mission is to integrate advanced technologies and talents from domestic and international sources for the research and development of new drugs. It possesses a technical platform for developing formulations of small molecule drugs and provides excellent GCP clinical trial monitoring services. Genovate also operates a production facility compliant with PIC/S GMP standards, manufacturing and selling high-quality pharmaceuticals.
To expand its product portfolio, Genovate has successfully completed a series of strategic investments, bringing in partners such as NaviFUS, Uni Pharma, and Soleno. These partnerships aim to enhance Genovate’s value and international competitiveness.
Positioning and vision
Specializing in the research and development of small molecule drugs, Genovate aims to become a distinctive pharmaceutical manufacturer focusing on “Niche in class” new drugs. Its strategy involves collaborating with experts, industry partners, and government entities to develop a balanced portfolio of products, ensuring steady growth while seizing opportunities for advancement.
Features and Advantages
Genovate boasts extensive capabilities and experience in new drug development, spanning from raw material production to human clinical trials and FDA approval processes. As Taiwan’s domestic drug development environment matures, Genovate has begun outsourcing certain tasks such as drug synthesis, biochemical analysis, and human PK trials. However, maintaining in-depth knowledge of these technologies is crucial to ensuring the quality and effectiveness of outsourced work.
The company’s strengths include its track record in executing numerous government initiatives, establishing robust project management capabilities, and fostering strong collaborations with industry, government, academia, and research institutions. Since its listing in 2012, Genovate has embarked on a series of strategic investments, successfully integrating partners with international competitive advantages like NaviFUS, Uni Pharma, and Soleno to tap into global markets through collaborative research and development.
New drug research & Development
Genovate retains all “soft power” in drug development (market analysis, drug design, project management, regulatory patents) and some “hard skill” (formulation development, drug production). This is in line with the company’s business strategy and outsourced research and development efforts to develop three types of drugs for long-term, medium-term, and short-term gains:
(1)
New chemical entities: Developed through industry alliances and government subsidy programs, with the goal of completing early-stage human clinical trials, such as DBPR108. Upon achieving interim milestones, strategic partners will take over development under licensing agreements.
(2)
New pathways and formulations: Initial registration in the domestic market is the primary objective, followed by further development for international markets, such as PMR and GV17.
(3)
Branded generics: Overcoming patent and formulation challenges or conducting small-scale human clinical trials, targeting niche markets as the first to market with a generic version.
PIC/S GMP production
Genovate is dedicated to the development of new drugs. To meet the stringent requirements of drug development, it has invested in acquiring the GMP facility of Original Bristol-Myers Squibb Company and continues to update its existing facilities and machinery. Genovate also recruits professional talent and aims to adhere to the cGMP standards set by the US FDA to rigorously manage the quality of pharmaceutical production. Genovate not only manufactures its own research products but also acts as a contract manufacturer for Taiwan’s market for Daiichi Sankyo, TSH Biopharm, Roche, and several domestic distributors. Hukou Plant obtained approval from the Department of Health in December 2004 for “comprehensive completion of cGMP verification operations,” received approval from the Japanese Ministry of Health, Labour and Welfare as a certified foreign pharmaceutical manufacturer in December 2007, and passed the Department of Health (PIC/S) GMP verification operation audit in September 2010. The quality and capabilities of Genovate products are widely recognized in the industry.
Business and Marketing
Genovate’s sales team not only markets its own products but also strengthens its marketing capabilities through strategic alliances, achieving a balanced financial performance since 2003. Its market channels include medical centers, regional hospitals, clinics, and an expanding export market across Southeast Asia since 2001. Moreover, Genovate has been introducing products from European and American pharmaceutical firms since 2003.
In recent years, Genovate has diversified into other biomedical fields beyond small molecule drugs through investments and strategic alliances, exploring areas such as innovative medical devices, pet cancer screening, and cell therapy, aiming for diversified and multi-faceted development.
In conclusion, Genovate is recognized for its robust R&D capabilities, comprehensive product pipeline, and stringent quality control, making it a prominent player not only in the Taiwanese market but also in the competitive landscape of global pharmaceutical development and manufacturing.
Company History
1995 |
Received investment from the National Development Fund, Executive Yuan, becoming the first government-supported flagship biopharmaceutical company. |
1997 |
Acquired the Original Bristol-Myers Squibb GMP facility, established the clinical trial department, and introduced CRO services. |
2000 |
Completed Phase III clinical trials for Prestara™, a treatment for SLE, and submitted a New Drug Application (NDA) to the US FDA. Received an “Approvable Letter” from the US FDA in 2002. |
2001 |
Collaborated with Roche to develop and manufacture the antiviral drug Ribavirin. |
2003 |
Launched new drugs: “Genetaxyl” (paclitaxel injection) and “Urotrol” (urinary incontinence treatment).Listed on the Emerging Stock Market (OTC), breaking even in the first year. |
2004 |
Licensed Tanabe in Japan and Yuhan in Korea to develop Prestara™, receiving upfront licensing fees. |
2008 |
Collaborated with the National Health Research Institutes to jointly develop the anti-diabetes drug DBPR108. Granted market authorization to CSPC Pharmaceutical Group in China in 2012. |
2009 |
Signed a contract manufacturing agreement with Taiwan’s Daiichi Sankyo. |
2010 |
Factory passed Department of Health PIC-S/GMP certification. Approved for the manufacture of sterile ophthalmic solutions in 2014.。 |
2012 |
Listed on the Taipei Exchange (TPEx) (stock code: 4130). |
2013 |
Invested in SyneuRx. Assisted CMC in completing SND11 and SND13 (2014) IND filings for the US, entering Phase II/III clinical trials.Invested in Reber Genetics (stock code: 6479) and THEVAX, acquiring joint development rights for the HPV Vx Chinese market. |
2014 |
Phase II clinical results of PMR met expectations. Licensed to Otsuka Pharmaceuticals in Asia-Pacific for joint development.Granpatch entered registration clinical trials. |
2015 |
Colon cleanser Bowklean approved by TFDA. PMR entered Phase III clinical trials.Invested in US cell immunotherapy company Medeor. |
2016 |
Invested in NaviFUS, using non-invasive focused ultrasound to temporarily open the blood-brain barrier, providing treatment options for CNS-related diseases. |
2017 |
Invested in Soleno, developing a new drug for Prader-Willi Syndrome (PWS). Invested in GenVax, developing distinctive new drug formulations.PMR completed US IND application and initiated human trials in the US. |
2018 |
Received TFDA approval for Mycocep, a new drug for lupus nephritis. |
2019 |
PMR completed pivotal clinical trials in the US.Co-invested with NaviFUS in GNI, launching the NF02 innovative medical device project for Alzheimer’s disease treatment in Melbourne, Australia. |
2020 |
PMR completed US Pre-NDA meeting and GV17 Pre-IND meeting application.The new drug DBPR108 for the treatment of diabetes has entered the third phase of clinical trials in mainland China.PMR obtained a patent in mainland China. |
2022 |
The new drug GX17 was developed for the treatment of “antiphospholipid syndrome (APS)” indications. It passed review by the US FDA and was approved for the first human clinical trial. |
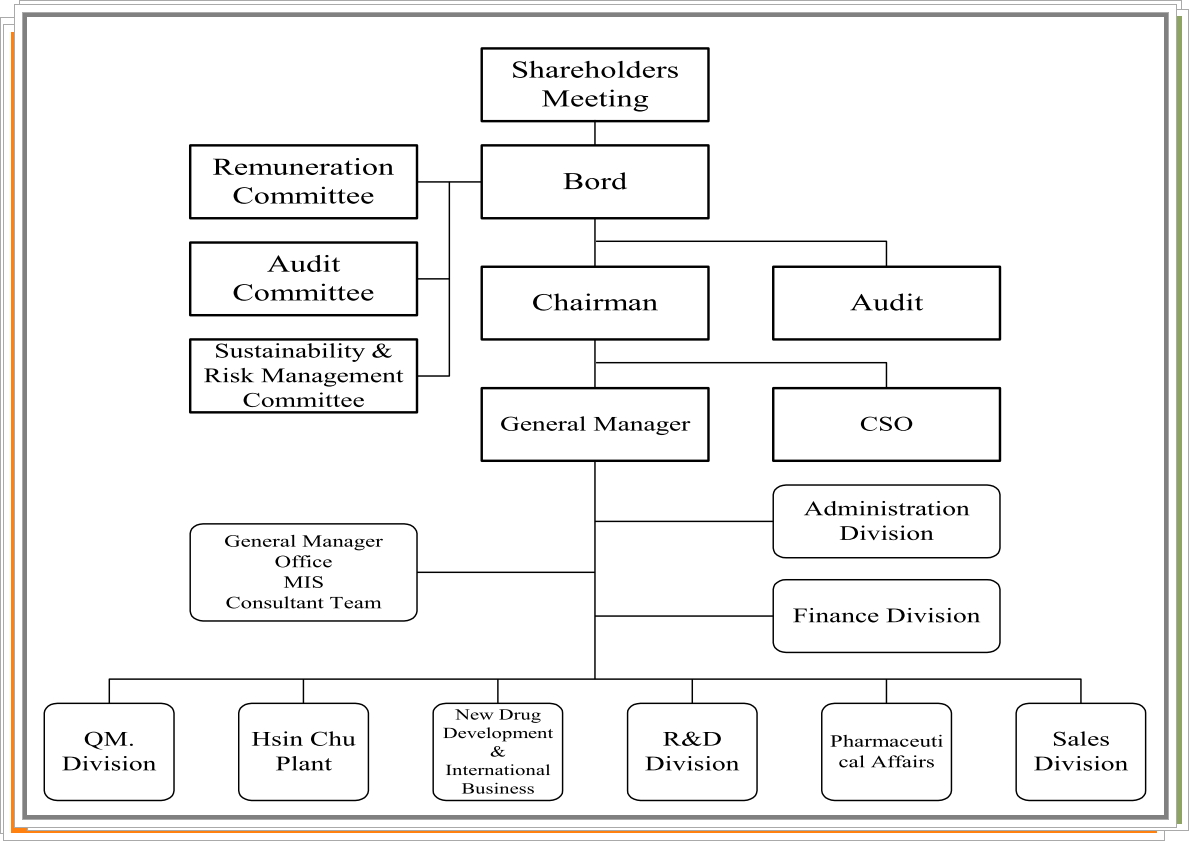
Management
Title |
Name |
Experience (Education) |
Other Position |
Chairman/ Chief Strategy Officer |
Chen, Jen |
.Ph.D. in Chemistry, University of Rochester, USA.Novartis Program Host.Genelabs, Vice Chairman of Asia Sales.QPS-Qualitix Clinical Research Co., Ltd., Chairman.Bio Taiwan Committee (BTC), Executive Yuan, Committee Member |
.Genovate Biotechnology Co., Ltd., Chairman and CSO.Quest Pharmaceutical Services Taiwan Co., Ltd., Corporate Representative (Director).Genovate Biotechnology (Cayman) Co., Ltd., Corporate Representative (Director).Unipharma Co. Ltd., Chairman and CSO.Reber Genetics Co., Ltd., Corporate Representative (Director).NaviFUS Corporation, corporate representative (Chairman) and CSO.Genovate NaviFus (Australia)Pty. Ltd., Corporate Representative (Director).Savior Lifetec Corporation, Corporate Representative (Director) |
General Manager |
Chu,Chia-Chen |
.Master’s Degree, Science in Health Policy and Management, Harvard University.Ministry of Health and Welfare, Associate Researcher.Genovate Biotechnology Co., Ltd., Director of International Affairs.Genovate Biotechnology Co., Ltd., New Drug Development Department, Vice President |
.Unipharma Co. Ltd., Corporate Representative (Director).NaviFUS Corporation, Corporate Representative (Director).Genovate NaviFus Inc., Corporate Representative (Director).Genovate NaviFus (Australia)Pty.Ltd., Corporate Representative (Director) |
Vice President, Finance |
Lin,Hui-Ling |
.Master of Institute of Finance, National Yang Ming Chiao Tung University.Deloitte Touche Tohmatsu Limited, Accounting Firm Deputy Supervisor.Genovate Biotechnology Co., Ltd., Audit Manager.Genovate Biotechnology Co., Ltd., Finance Division Director |
None |
Senior Director, AdministrationDivision |
Chiang, Wei-Min |
.Master of Institute of Biology, National Taiwan Normal University.Fair Friend Enterprise Co., Ltd., Assistant Manager.Dalson Education group, HR Director |
None |
Audit Supervisor |
Chan,Ya-Lan |
.Graduated from Department of Accounting, Tamkang University.IC PricewaterhouseCoopers Taiwan.Accounting Supervisor, Genovate Biotechnology Co., Ltd. |
None |
Plant Director |
Huang, Kuang-Liang |
.Graduated from Shih Hsin University, Department of Printing and Photography..Production Section Chief at Panion & BF Biotech Inc..Deputy Plant Manager at Purzer Pharmaceutical Co., Ltd. |
None |

 繁中
繁中